OXYGEN THERAPY
Health Canada and the Food and Drug Act
According to the Food and Drug Act:
a “drug” includes any substance or mixture of substances manufactured, sold or represented for use in:
Did You Know?
Health Canada administers the Food and Drug Act.
Once a drug has been authorized, Health Canada issues an eight-digit Drug Identification Numbers (DIN) which permits the manufacturer to market the drug in Canada.
Health Canada sets the standards and guidelines for the manufacturing of drugs and health products (including medical gases such as oxygen) to ensure they are safe for human and veterinary use.
In Canada, medical oxygen containers and systems require proper labels which include DINs.
An Overview of the Phases of Drug Action
adapted from (Rau, J.L., 2002. p. 13)
Drug Administration
Pharmacokinetic Phase
Pharmacodynamic Phase
Effect
Indications for Oxygen Therapy
Documented hypoxemia, defined as a decreased PaO2 in the blood below normal range. PaO2 of < 60 torr or SaO2 of < 90% in patients breathing room air, or with PaO2 and/or SaO2 below desirable range for specific clinical situation. Clinical acceptable ranges may depend on patient age, condition and/or disease process.
Did You Know?
The evidence-based approach to the treatment of COPD with oxygen is ever evolving. The American Thoracic Society released a new set of guidelines in 2020:
New COPD Oxygen Therapy Guidelines
“Oxygen is a treatment for hypoxemia, not breathlessness. Oxygen has not been proven to have any consistent effect on the sensation of breathlessness in non-hypoxemic patients.” (BTS 2017)
Absolute Contraindications & Possible Adverse Effects
Absolute Contraindications
The use of some O2 delivery devices (e.g., nasal cannulas and nasopharyngeal catheters in neonates and pediatric patients that
have nasal obstructions)
Potential Adverse Effects
Absorption atelectasis
Goals of Oxygen Therapy
“Oxygen Therapy is usually defined as the administration of oxygen at concentrations greater than those found in ambient air” (BTS, 2011. p.vi27).
The main goal of oxygen therapy is:
“To treat or prevent hypoxemia thereby preventing tissue hypoxia which may result in tissue injury or even cell death” (BTS, 2011. p.vi27).
Hypoxia
Did You Know?
Hypoxia can exist even though hypoxemia has been corrected with oxygen therapy?
For example:
• At the cellular level where the cells are unable to access or use the O2 delivered
• At the tissue level when O2 may not reach the cells due to a blocked artery
The causes of hypoxia are (BTS, 2011, p.vi14):
• Hypoxemia (e.g., at high altitudes).
• Anemic hypoxemia (e.g., reduced hematocrit or carbon monoxide poisoning).
• Stagnant hypoxemia (e.g., shock, ischemia).
• Histotoxic hypoxia/dysoxia (e.g., cyanide poisoning).
Hypoxemia
If the partial pressure of O2 (PaO2) is less than the level predicted for the individual’s age, hypoxemia is said to be present.
Some of the causes of hypoxemia are:
Low Pinspired O2 (e.g., at high altitude).
Hypoventilation, V/Q mismatch (e.g., COPD).
Anatomical Shunt (e.g., cardiac anomalies).
Physiological Shunt (e.g., atelectasis).
Diffusion deficit (e.g., interstitial lung disease).
Hemoglobin deficiencies.
Did You Know?
In Ontario, the MOHLTC sets guidelines defining hypoxemia and the criteria for long-term use of oxygen.
The criteria are:
- Each applicant’s condition must be stabilized and treatment regimen optimized before long-term oxygen therapy is considered. Optimum treatment includes smoking cessation.
- Applicants must have chronic hypoxemia on room air at rest (Pa02 of 55mmHg or less, or Sa02 of 88% or less).
- Applicants with persistent Pa02 in the range of 56 to 60 mmHg may be considered candidates for long-term oxygen therapy if any of the following medical conditions are present:
-
- cor pulmonale;
- pulmonary hypertension; or
- persistent erythrocytosis.
-
Also, some applicants with a persistent Pa02 in the range of 56 to 60mmHg may be candidates for long-term oxygen therapy if the following occurs:
- exercise limited hypoxemia;
- documented to improve with supplemental oxygen;
- nocturnal hypoxemia.
Retrieved from: www.health.gov.on.ca/en
There is Medical Eligibility Criteria for exertional hypoxemia, as well as special considerations for patients diagnosed with pulmonary fibrosis.
The Effects of Hypoxia and Hyperoxia
(O’Driscoll, 2008)
Hypoxia
| Effects | Risks | |
|---|---|---|
| Respiratory System | - Increased ventilation - Pulmonary vasoconstriction | - Pulmonary hypertension |
| Cardiovascular system | - Coronary vasodilation - Decreased systemic vascular resistance (transient) - Increased cardiac output - Tachycardia | - Myocardial ischemia/infarction - Ischemia/infarction of other critically perfused organs - Hypotension - Arrhythmias |
| Metabolic system | - Increased 2,3-DPG - Increased CO2 carriage (Haldane effect) | - Lactic acidosis |
| Neurological system | - Increased cerebral blood flow due to vasodilation | - Confusion - Delirium - Coma |
| Renal system | - Renin-angiotensin axis activation - Increased erythropoietin production | - Acute tubular necrosis |
Hyeroxia
| Effects | Risks | |
|---|---|---|
| Respiratory System | - Decreased ventilation | - Worsened ventilation / perfusion matching - Absorption atelectasis |
| Cardiovascular system | - Myocardial ischemia (in context of decreased haematocrit) - Reduced cardiac output - Reduced coronary blood flow - Increased blood pressure - Increased reactive oxygen species |
|
| Metabolic system | - Decreased 2,3-DPG - Decreased CO2 carriage (Haldane effect) | - Increased reactive oxygen species |
| Neurological system | - Decreased cerebral blood flow | |
| Renal system | - Reduced renal blood flow |
Drive to Breathe and Carbon Dioxide Retention
The primary goal of oxygen therapy is to treat hypoxemia. However, a very small number of patients with Chronic Obstructive Pulmonary Disease (COPD) have sensitivity to higher levels of O2.
Target saturation for patients at risk of hypercapneic respiratory failure is 88-92% (BTS, 2016) unless otherwise prescribed, pending blood gas results.
If you are unsure if a patient has a sensitivity to O2, the main goal is to treat hypoxemia.
For more information on best practice guidelines for the treatment of COPD please visit the Canadian Thoracic Society – COPD Guideline Library website.
Emphasis is always to avoid harmful hypoxemia and hypercapnia by carefully titrating O2 and monitoring arterial blood gases.
Did You Know?
Normal range of Carbon Dioxide (CO2) is generally accepted as 35-45 mmHg.
Normally, increased levels of CO2 will stimulate ventilation. Patients with certain respiratory diseases such as COPD may have reduced sensitivity to increased levels of CO2.
Hypoxic drive refers to the patient being dependent on low levels of arterial blood oxygen (PaO2) to stimulate breathing as seen is some patients with COPD.
If too much O2 is given to a patient who relies on hypoxic drive to breathe, the blood oxygen levels will rise but the CO2 level will rise as well, leading to respiratory acidosis and failure.
How Does Oxygen Therapy Work?
Oxygen Transport
Oxygen carried in the blood is reversibly bound to the hemoglobin. A very tiny amount of free oxygen gas dissolved in the plasma. Dissolved oxygen gas exerts a pressure in the vasculature that can be measured from a blood sample (e.g., an arterial blood gas (ABG)). This measurement is known as the partial pressure of oxygen in the arterial blood and is represented by the nomenclature: PaO2.
The majority of oxygen carried in the blood is transported bound to hemoglobin. A very small amount of oxygen gas is transported dissolved in the plasma. This dissolved O2 can be measured utilizing a small sample of arterial blood. This measurement is referred to as PaO2 and is an important indicator when assessing for hypoxia.
Oxygen Hemoglobin Dissociation Curve
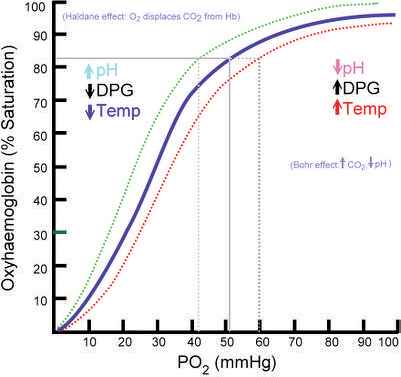
The oxyhemoglobin dissociation curve is tool for understanding how our blood carries and releases oxygen. In the oxyhemoglobin dissociation curve, oxygen saturation (SO2) is compared to the partial pressure of oxygen in the blood (PO2), and this creates a curve that demonstrates how readily hemoglobin acquired and releases oxygen molecules into the fluid that surrounds it (oxygen- hemoglobin affinity).
Some of the factors affecting the loading and unloading of oxygen are:
- Blood pH (Bohr effect)
- Body temperature
- Erythrocyte concentration of certain organic phosphates (e.g., 2,3 diphosphoglycerate)
- Variation to the structure of the hemoglobin (Hb) molecules (e.g., sickle cells, methemoglobin (metHb) and fetal hemoglobin (HbF))
- Chemical combinations of Hb with other substances (e.g., carbon monoxide)
Remember, changes to and of these factors may cause the oxygen dissociation curve to shift right or left; affecting the oxygen-hemoglobin affinity.
Gas Exchange of Oxygen
Oxygen Cascade
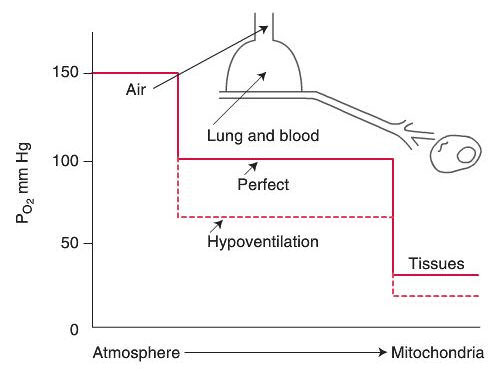
Alveolar Air Equation
PAO2 = [(PB-PH2O) * FiO2] – PaCO2 /RQ
Normal Diffusion of Oxygen
With regards to diffusion of oxygen in the normal lung at Body Temperature and Pressure Saturated (BTPS):
The partial pressure of oxygen in the alveolus (PAO2) approximates 100mmHg.
The partial pressure of oxygen in the venous blood returning to the lung (PVO2) approximates 40mmHg, there is a pressure gradient for diffusion of oxygen into the blood of about 60 mmHg.
Theoretically, the partial pressure in the capillary blood should rise to equal the partial pressure of oxygen in the alveolus and therefore the partial pressure of oxygen in the arterial blood (PaO2) should approximate 100mmHg “the PaO2of healthy individuals breathing air at sea level is always approximately 5-10 mmHg less than the calculatedPaO2. Two factors account for this difference: (1) right to left shunts in the pulmonary and cardiac circulation, and (2) regional differences in the pulmonary ventilation and blood flow” (Kacmarek, Stoller, Heuer,2013, p. 255). Normal PaO2 is expected to range from 90-95mmHg however, in clinical practice normoxemia in adults and children is defined as 80-100 mmHg.
Neonates have a lower actual PaO2 than adults and children. In
neonates normoxemia is 50-80mmHg due to anatomical shunts at birth
and the nature of fetal hemoglobin.
At the tissue level, oxygen diffuses from the blood (Pcapillaries O2= 40 mmHg) across the microvasculature and interstitial space into the cell (Pintracellular O2= 5mmHg) where cellular respiration take place. The movement of gas across the alveolar-capillary membrane is best described by Fick’s first law of diffusion.
Fick’s Law of Diffusion
V = A x D (P1 – P2)
T
Where the factors affecting gas exchange are:
V = flow of gas (oxygen)
A = cross sectional area available for diffusion
D = diffusion coefficient
P1 – P2 = the partial pressure gradient
P1 = partial pressure of oxygen in the alveolus (PAO2)
P2 = partial pressure of oxygen in the blood (PaO2)
T = thickness of the membrane (alveolar-capillary membrane)
Pathophysiological Factors Affecting Gas Exchange
Some of the pathophysiologic factors affecting the gas exchange of oxygen include:
the carrying content of the blood (SaO2 and PaO2) e.g. sickle cell anemia, carbon monoxide poisoning, hypoxemia;
GLOSSARY
(ATP) Ambient Temperature and Pressure = (STP) standard temperature and pressure = 0C and 1 atmosphere
BTPS = Body Temperature and ambient Pressure Saturated = 37 °C, 1 atmosphere, and 44 mg H2O/L
Conserving Devices - How long liquid and cylinder systems last before refilling depends on the amount of oxygen a person uses. Conserving devices extend the length of time. Oxygen systems deliver oxygen continuously during inspiration and exhalation. Conserving devices can be programmed to deliver oxygen during inspiration only, therefore reducing the amount wasted during exhalation.
Cryogenic Vessel - A static or mobile vacuum insulated container designed to contain liquefied gas at extremely low temperatures. Mobile vessels could also be known as "Dewars". Retrieved from: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/guidance-documents/gmp-guidelines-0031/document.html
Drug Identification Number (DIN) - a computer-generated eight-digit number assigned by Health Canada to a drug product prior to being marketed in Canada. It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter drug products that have been evaluated and authorized for sale in Canada. A DIN uniquely identifies the following product characteristics: manufacturer; product name; active ingredient(s); strength(s) of active ingredient(s); pharmaceutical form; route of administration. Retrieved from: www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/fs-fi/dinfs_fd-eng.php
Fractional Distillation - the process of separating the portions of a mixture by heating it and condensing the components according to their different boiling points. Retreived from: http://medical-dictionary.thefreedictionary.com/fractional+distillation
Medical gas - (either a single gas or a mixture of gases) is a gas that requires no further processing in order to be administered, but is not in its final package (e.g., liquefied oxygen) and is known as a bulk gas. Retrieved from: http://ccinfoweb2.ccohs.ca/legislation/documents/stds/csa/cmgpi12e.htm
Manifold (rampe) - Equipment or apparatus designed to enable one or more medical gas containers to be filled at a time.
REFERENCES
- American Thoracic Society (2020) Clinical Practice Guideline: Home Oxygen Therapy for Adults with Chronic Lung Disease. Retrieved from: https://www.atsjournals.org/doi/pdf/10.1164/rccm.202009-3608ST
- Becker, D. E., & Casabianca, A. B. (2009). Respiratory monitoring: physiological and technical considerations. Anesthesia Progress, 56(1), 14-20. doi: 10.2344/0003-3006-56.1.14.
- Cairo, J., M. & Pilbeam, S., P., (2017) Mosby’s Respiratory Care Equipment (10th ed.). St. Louis, MO: Mosby.
- Canadian Standards Association. (2016). Z305.12-06 (R2012) - Safe Storage, Handling, and Use of Portable Oxygen Systems in Residential Buildings and Health Care Facilities. Retrieved from: https://www.csagroup.org/store/search-results/?search=all~~Safe%20Storage,%20Handling,%20and%20Use%20of%20Portable%20Oxygen%20Systems%20in%20Residential%20Buildings%20and%20Health%20Care
- Cousins JL, Wark PA, McDonald VM. Acute oxygen therapy: a review of prescribing and delivery practices. Int J Chron Obstruct Pulmon Dis. 2016;11:1067-1075. Published 2016 May 24. doi:10.2147/COPD.S103607
- Gardenshire, D. (2020). Rau’s Respiratory Care Pharmacology. (10th ed.). St. Louis, MO: Mosby Inc.
- Kacmarek, R. M., Stoller, J.K. Heuer, A. J. (2021). Egan’s Fundamentals of Respiratory Care. (12th ed.). St. Louis, MO: Mosby.
- Mariciniuk, D. D., Goodridge, D., Hemandez, P., Rocker, J., Balter, M., Bailey, P., Brown, C. (2011). Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A Canadian Thoracic Society clinical practice guideline. Canadian Respiratory Journal, 18(2), 69–78. Retrieved from www.ncbi.nlm.nih.gov/pmc/articles/PMC3084418/
- Ministry of Health and Long-Term Care. Policy and Procedures Manual for the Assistive Devices Program (May 2016). Conflict of Interest. Retrieved from: Policies and Procedures Manual of the Assistive Devices Program (gov.on.ca)
- O'Driscoll, B. R., Howard, L. S., Earis, J., & Mak, V. (2017). British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ open respiratory research, 4(1), e000170. Retrieved from: https://doi.org/10.1136/bmjresp-2016-000170
- Sackett, D., Rosenberg, W., Gray, J., Haynes, R., & Richardson, W. (1996). Evidence-based medicine: what it is and what it isn't. British Medical Journal, 312, 71-72. Retrieved from: www.bmj.com/cgi/content/full/312/7023/71
