OXYGEN – A BRIEF REVIEW
Oxygen (O2) is the eighth element on the periodic table.
At ambient temperature and pressure (ATP), oxygen atoms bind together, sharing electrons to form molecules of oxygen that exist as a colourless, odourless transparent and tasteless gas with the chemical symbol O2.
Fast Facts about O2
Accelerates combustion.
At -182.9 C (-300 F) oxygen is a pale blue liquid.
Its critical temperature is -118.4 C (above this critical temperature oxygen can only exist as a gas regardless of the pressure).
An oxygen enriched environment is considered to have 23% oxygen in the air and is a fire hazard.
“Oxygen sustains life and supports combustion. While there are many benefits to oxygen by inhalation, it is not without hazards and toxic effects. It is therefore important for persons who are responsible for oxygen administration to be familiar with its indications for use, potential hazards and equipment” (Kacmarek, Stoller & Heuer, 2013).
Overview – Types of Oxygen Delivery Systems
There are three main types of oxygen delivery systems:
Compressed gas cylinders;
Liquid oxygen in cryogenic containers; and
Oxygen concentrators for medical use
Considerations for the selection of oxygen source include, but are not limited to, factors such as:
Size and weight of the device;
Storage capacity; and
Cost and the ability to fill the device
For a good comparison of portable oxygen source and delivery devices please visit the American Thoracic Society (ATS) website.
Did You Know?
The manufacturing and distribution of medical oxygen in Canada is primarily regulated by Health Canada who set the standards and guidelines for the manufacturing and distribution of drugs and health products (including medical gases such as oxygen). Their mandate is to ensure medical gases are safe for human and veterinary use.
Compressed Gas Cylinders
Oxygen is packaged and shipped as a high-pressure gas in seamless steel or aluminum cylinders constructed to Transport Canada and CSA specifications. In cylinders charged with gaseous oxygen, the pressure in the container is related both to temperature and the amount of oxygen in the container. Full high-pressure cylinders normally contain gas at 15 169 kPa (2200 psig) at 21 °C (70°F). Cylinder content can be determined by pressure, i.e., at a given temperature, when the gas pressure is reduced to half the original pressure, the cylinder will be approximately half full. The pressure of a full cylinder of oxygen is normally 2200 psig.
Bulk Oxygen
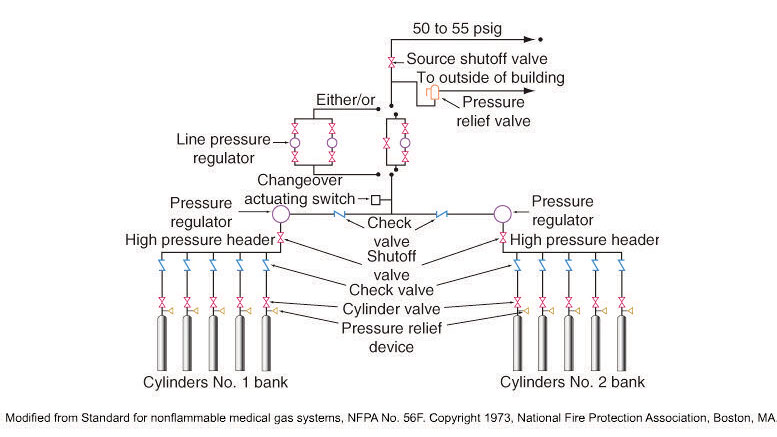
Cylinders may be used various ways. For example, in a manifold system, large sized cylinders are linked together to supply medical oxygen to medical gas pipelines which then lead directly to the bedside in hospitals.
Manifold System
Portable Oxygen Cylinders
Smaller sized cylinders are used as portable individual oxygen systems for short termuse.
Did You Know?
To calculate how long a cylinder will last based on the size of the cylinder and continuous flow rate, the following formula can be used:
Duration of Flow in minutes =
(gauge pressure psi – safe residual pressure psi) x cylinder factor
Flow rate in liters per minute
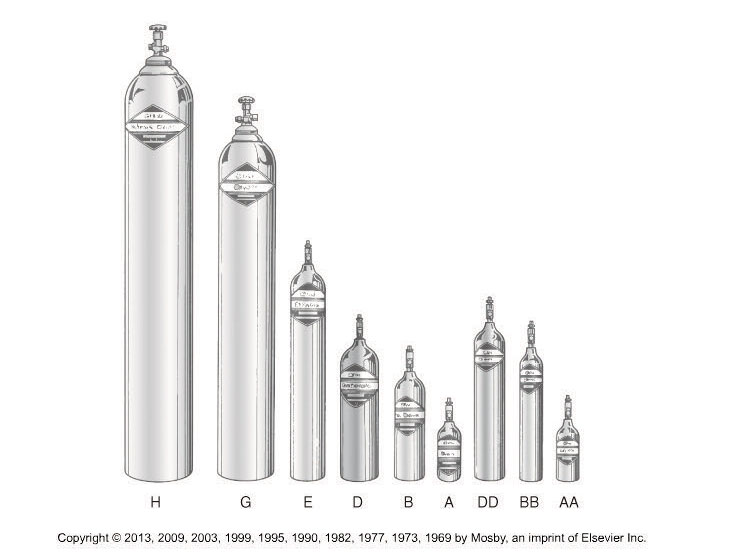
Some examples of cylinder factors for different sized cylinders are:
D cylinder 0.16
E cylinder 0.28
M cylinder 1.56
H cylinder 3.14
Liquid Oxygen in Cryogenic Containers
Bulk Liquid Oxygen systems
Liquid oxygen can be manufactured by fractional distillation of air at an oxygen manufacturing plant and then delivered and stored on site to supply the healthcare facility. In this case, large stores of liquid oxygen are referred to as bulk oxygen. The oxygen is stored on site in large cryogenic vessels known as dewars. These dewars are regularly refilled by the oxygen gas manufacturer/supplier.
As the liquid oxygen passes through warming coils and is allowed to evaporate, the gas is delivered to a medical gas pipeline system and then directly to the bedside.

Portable Liquid Oxygen
Various sizes of smaller, base-unit, cryogenic containers (also known as reservoirs) can be used in various settings such as long-term care facilities, homes, and hospital wards to fill smaller portable cryogenic liquid systems that patients can ambulate with. Portable liquid oxygen units offer continuous flow or intermittent flow of oxygen to the patient/client.
The Canadian Standards Association (CSA) offers standards and guidelines on the safety, storage, and delivery of liquid oxygen.
Oxygen Concentrators for Medical Use
Oxygen concentrators provide a safe source of oxygen-enriched air. They are devices which employ selective removal of nitrogen from room air to increase the concentration of oxygen in the delivered gas product. A concentrator is an electrical or battery powered, electronically controlled device that does not store oxygen when not in operation.
Industrial sized oxygen concentrators can supply oxygen to their medical gas pipelines systems which is then delivered directly to the bedside. Concentrators use one of two main methods to separate and concentrate oxygen from the air, molecular sieves or semi-permeable membranes.
Molecular sieves use sodium-aluminum silicate crystals and employ Pressure Swing Adsorption (PSA) or Vacuum Pressure Swing Adsorption (VPSA) technology.
Semi-permeable membranes are thin plastic membranes that are selectively permeable to O2 molecules and water vapor.
Did You Know?
Battery-operated portable oxygen concentrators can function in continuous flow mode and / or pulse dose / demand mode.
Portable Oxygen Supply
Smaller individual concentrators can provide oxygen at a hospital bedside, in the home or on the go. They also separate oxygen from air using molecular sieves or semi-permeable membranes.
There are three types:
Stationary concentrators;
Concentrators that have the ability to fill portable aluminum cylinders; and
Portable oxygen concentrators that operate using lithium batteries

Oxygen Concentrator
Oxygen Safety at Home
The CSA has developed standards related to the safe storage, handling and use of portable oxygen systems in residential and healthcare facilities. This is a key resource and includes input from CRTO Members from across Ontario.
The Canadian Centre for Occupational Health and Safety also has several resources that are available to the public. You can visit the Canadian Centre for Occupational Health and Safety website and enter the search term ‘oxygen’ to find out more. Here are some links of interest:
Compressed Gases Hazards
www.ccohs.ca/oshanswers/chemicals/compressed/compress.html
Storage and Handling of Compressed Gas Cylinders
www.ccohs.ca/oshanswers/safety_haz/welding/storage.html
Working with Compressed Gases
www.ccohs.ca/oshanswers/prevention/comp_gas.html
How Do I Work Safely with Cryogenic Liquids?
www.ccohs.ca/oshanswers/prevention/cryogens.html#_1_7
- Transport Canada – Transportation of dangerous goods in Canada – Oxygen is a Class 2.2, Non-flammable, Non-toxic gases.
- Health Canada – Workplace Hazardous Materials Information System (WHMIS).
Oxygen is a Class A: compressed gas.
Manufacturers of therapeutic oxygen in Canada are responsible for providing WHMIS Material Safety Data Sheets (MSDS) for oxygen and may be found on their websites.
GLOSSARY
(ATP) Ambient Temperature and Pressure = (STP) standard temperature and pressure = 0C and 1 atmosphere
BTPS = Body Temperature and ambient Pressure Saturated = 37 °C, 1 atmosphere, and 44 mg H2O/L
Conserving Devices - How long liquid and cylinder systems last before refilling depends on the amount of oxygen a person uses. Conserving devices extend the length of time. Oxygen systems deliver oxygen continuously during inspiration and exhalation. Conserving devices can be programmed to deliver oxygen during inspiration only, therefore reducing the amount wasted during exhalation.
Cryogenic Vessel - A static or mobile vacuum insulated container designed to contain liquefied gas at extremely low temperatures. Mobile vessels could also be known as "Dewars". Retrieved from: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/guidance-documents/gmp-guidelines-0031/document.html
Drug Identification Number (DIN) - a computer-generated eight-digit number assigned by Health Canada to a drug product prior to being marketed in Canada. It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter drug products that have been evaluated and authorized for sale in Canada. A DIN uniquely identifies the following product characteristics: manufacturer; product name; active ingredient(s); strength(s) of active ingredient(s); pharmaceutical form; route of administration. Retrieved from: www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/fs-fi/dinfs_fd-eng.php
Fractional Distillation - the process of separating the portions of a mixture by heating it and condensing the components according to their different boiling points. Retreived from: http://medical-dictionary.thefreedictionary.com/fractional+distillation
Medical gas - (either a single gas or a mixture of gases) is a gas that requires no further processing in order to be administered, but is not in its final package (e.g., liquefied oxygen) and is known as a bulk gas. Retrieved from: http://ccinfoweb2.ccohs.ca/legislation/documents/stds/csa/cmgpi12e.htm
Manifold (rampe) - Equipment or apparatus designed to enable one or more medical gas containers to be filled at a time.
REFERENCES
- American Thoracic Society (2020) Clinical Practice Guideline: Home Oxygen Therapy for Adults with Chronic Lung Disease. Retrieved from: https://www.atsjournals.org/doi/pdf/10.1164/rccm.202009-3608ST
- Becker, D. E., & Casabianca, A. B. (2009). Respiratory monitoring: physiological and technical considerations. Anesthesia Progress, 56(1), 14-20. doi: 10.2344/0003-3006-56.1.14.
- Cairo, J., M. & Pilbeam, S., P., (2017) Mosby’s Respiratory Care Equipment (10th ed.). St. Louis, MO: Mosby.
- Canadian Standards Association. (2016). Z305.12-06 (R2012) - Safe Storage, Handling, and Use of Portable Oxygen Systems in Residential Buildings and Health Care Facilities. Retrieved from: https://www.csagroup.org/store/search-results/?search=all~~Safe%20Storage,%20Handling,%20and%20Use%20of%20Portable%20Oxygen%20Systems%20in%20Residential%20Buildings%20and%20Health%20Care
- Cousins JL, Wark PA, McDonald VM. Acute oxygen therapy: a review of prescribing and delivery practices. Int J Chron Obstruct Pulmon Dis. 2016;11:1067-1075. Published 2016 May 24. doi:10.2147/COPD.S103607
- Gardenshire, D. (2020). Rau’s Respiratory Care Pharmacology. (10th ed.). St. Louis, MO: Mosby Inc.
- Kacmarek, R. M., Stoller, J.K. Heuer, A. J. (2021). Egan’s Fundamentals of Respiratory Care. (12th ed.). St. Louis, MO: Mosby.
- Mariciniuk, D. D., Goodridge, D., Hemandez, P., Rocker, J., Balter, M., Bailey, P., Brown, C. (2011). Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A Canadian Thoracic Society clinical practice guideline. Canadian Respiratory Journal, 18(2), 69–78. Retrieved from www.ncbi.nlm.nih.gov/pmc/articles/PMC3084418/
- Ministry of Health and Long-Term Care. Policy and Procedures Manual for the Assistive Devices Program (May 2016). Conflict of Interest. Retrieved from: Policies and Procedures Manual of the Assistive Devices Program (gov.on.ca)
- O'Driscoll, B. R., Howard, L. S., Earis, J., & Mak, V. (2017). British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ open respiratory research, 4(1), e000170. Retrieved from: https://doi.org/10.1136/bmjresp-2016-000170
- Sackett, D., Rosenberg, W., Gray, J., Haynes, R., & Richardson, W. (1996). Evidence-based medicine: what it is and what it isn't. British Medical Journal, 312, 71-72. Retrieved from: www.bmj.com/cgi/content/full/312/7023/71
