SPECIAL CONSIDERATIONS
Neonatal Care
Providing oxygen therapy to the neonatal population is complex and based on each individual clinical situation. For the immediate newborn period, it is generally accepted that oxygen is provided based on the American Academy of Pediatrics’ Textbook of Neonatal Resuscitation using the Canadian Adaptations from Canadian Pediatric Society.
Resources:
Canadian Pediatric Society (2020). 7th Edition NRP Guidelines.
Retrieved from: https://cps.ca/en/nrp-prn/faqs#Oxygen%20Administration
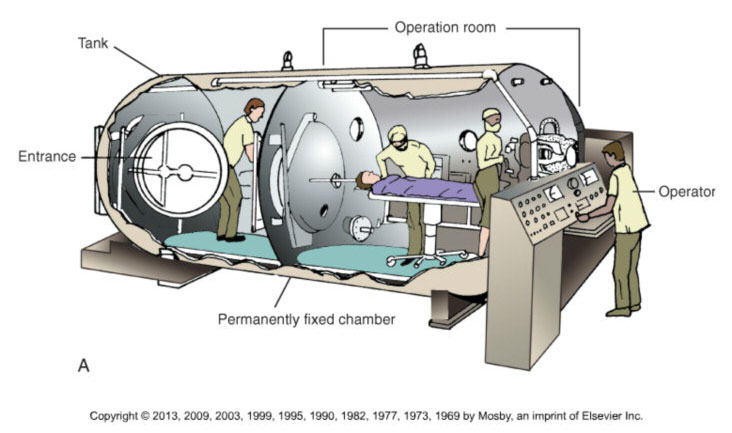
Hyperbaric Oxygen Therapy (HBOT)
The Basic Principles of Operation of Hyperbaric Chambers
The increased pressure inside the chamber, combined with the delivery of 100% oxygen (FiO2 = 1.0), drives the diffusion of oxygen into the blood plasma at up to 10 times normal concentration. Patients are monitored at all times during HBOT, often by RTs.
Did You Know?
The Undersea and Hyperbaric Medical Society (UHMS) is an international, nonprofit organization and is generally considered a primary source of scientific information for diving and hyperbaric medicine physiology worldwide.
Health Canada refers to UHMS guidelines and the CSA sets the standards for hyperbaric therapy in Canada.
Physiologic Effects of Hyperbaric Oxygen Therapy
aids the treatment of infection by enhancing white blood cell action and potentiating germ-killing antibiotics.
Indications
As of 2019, the following indications are approved uses of hyperbaric oxygen therapy as defined by the Undersea & Hyperbaric Medical Society (UHMS):
2. Carbon Monoxide Poisoning
4. Crush Injury, Compartment Syndrome and Other Acute Traumatic Ischemias
14. Idiopathic Sudden Sensorineural Hearing Loss*
(*approved on October 8, 2011 by the UHMS Board of Directors)
Potential Complications of Hyperbaric Oxygen Therapy
Barotrauma:
Ear or sinus trauma
Oxygen Toxicity:
Other:
The Safety of Hyperbaric Chambers (Health Canada)
Hyperbaric chambers are class 3 medical devices which must be licensed by Health Canada before they can be imported and sold in Canada. The Medical Devices Regulations require that the medical devices imported and sold in Canada are safe, effective, and of quality manufacture. This is achieved by a combination of a premarket review prior to licensing, and post-market surveillance of adverse events.
Health Canada has reviewed the scientific evidence related to hyperbaric chambers. The evidence shows that chambers are effective in treating at this time the 14 conditions recognized by the Undersea and Hyperbaric Medical Society. Therefore, Health Canada has issued medical device licences for hyperbaric chambers to treat only these 14 conditions. No device licences have been issued for the use of hyperbaric chambers to treat other conditions.
Undersea and Hyperbaric Medical Society
http://membership.uhms.org/?page=Indications
Undersea and Hyperbaric Medical Society Canadian Chapter
https://cuhma.ca/
University of Toronto Hyperbaric Medicine
(educational resource for healthcare professionals)
https://www.uhn.ca/Surgery/Treatments_Procedures/Hyperbaric_Medicine_Unit
Altitude Effects on the Availability of Oxygen
As altitude increases, barometric pressure decreases. Barometric pressure is the pressure that is exerted by the gases at a given point in the atmosphere, and is the sum of the partial pressures of the component gases. The composition of the atmosphere does not change with altitude, however, the barometric pressure does. As altitude increases, there is a decrease in the partial pressure exerted by each component gas. Thus, as altitude increases, the partial pressure of oxygen in the alveoli decreases. A reduced partial pressure of oxygen results in a relative hypoxia.
The following document is helpful for those planning travel by commercial airline:
Transport Canada – “Passengers with Medical Oxygen”
https://tc.canada.ca/en/aviation/reference-centre/advisory-circulars/advisory-circular-ac-no-700-002#s4-1
It is also helpful to consult the website of the specific airline that the patient intends to travel with for assistance in planning/arranging air travel when oxygen is required.
Did You Know?
In the cabin of a typical commercial aircraft, the pressure exerted is equivalent to the barometric pressure at 5000 – 8000 feet above sea level. As a result, patients who require oxygen supplementation at the ground level may require increased supplementation at an increased altitude.
Hyperbaric Oxygen & the 5th Authorized Act
For some time now, RTs have administered therapeutic oxygen in a hyperbaric practice setting under the 4th authorized act (“administering a substance by injection of inhalation”). As previously mentioned, the Public Hospitals Act still requires RTs to obtain an order from a valid authorizer to administer oxygen in this environment. As such, there is no change to the existing practices in hyperbaric settings within hospitals in Ontario.
The 5th authorized act, in combination with the Prescribed Substances regulation, now permits RTs to independently administer therapeutic oxygen. This means that in a hyperbaric setting outside of a hospital, RT s can administer oxygen without the additional requirement of an order for the oxygen from a physician or other authorizer. Administration of Hyperbaric Oxygen Therapy (HBOT), however, must occur in accordance with a diagnosis, pre-treatment screening and prescribed treatment profile (e.g., dive depth/pressure, time, etc.) that have been established by the most responsible physician (MRP). Therefore, RTs cannot independently initiate HBOT, but can implement this treatment in collaboration with the MRP.
HBOT is considered to be within the scope of practice of respiratory therapy; however, it requires competencies that are beyond those that an RT would possess at an entry-to-practice (i.e., graduate) level. In both the hospital and community setting, obtaining credentials as a Certified Hyperbaric Technologist (CHT) from the Undersea and Hyperbaric Medical Society (UHMS) is considered the industry standard, and is the benchmark that any RT administering hyperbaric oxygen would be expected to perform to.
As noted above, the CRTO has endorsed the list of 14 indications for hyperbaric oxygen therapy that are established by the UHMS. Health Canada supports the application of HBOT that is based on the UHMS guidelines and warns against “off label” uses that have not been scientifically proven to be effective. The CRTO does not endorse “off label” use of hyperbaric therapy and the engagement of an RT in such activity may be considered professional misconduct.
GLOSSARY
(ATP) Ambient Temperature and Pressure = (STP) standard temperature and pressure = 0C and 1 atmosphere
BTPS = Body Temperature and ambient Pressure Saturated = 37 °C, 1 atmosphere, and 44 mg H2O/L
Conserving Devices - How long liquid and cylinder systems last before refilling depends on the amount of oxygen a person uses. Conserving devices extend the length of time. Oxygen systems deliver oxygen continuously during inspiration and exhalation. Conserving devices can be programmed to deliver oxygen during inspiration only, therefore reducing the amount wasted during exhalation.
Cryogenic Vessel - A static or mobile vacuum insulated container designed to contain liquefied gas at extremely low temperatures. Mobile vessels could also be known as "Dewars". Retrieved from: https://www.canada.ca/en/health-canada/services/drugs-health-products/compliance-enforcement/good-manufacturing-practices/guidance-documents/gmp-guidelines-0031/document.html
Drug Identification Number (DIN) - a computer-generated eight-digit number assigned by Health Canada to a drug product prior to being marketed in Canada. It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter drug products that have been evaluated and authorized for sale in Canada. A DIN uniquely identifies the following product characteristics: manufacturer; product name; active ingredient(s); strength(s) of active ingredient(s); pharmaceutical form; route of administration. Retrieved from: www.hc-sc.gc.ca/dhp-mps/prodpharma/activit/fs-fi/dinfs_fd-eng.php
Fractional Distillation - the process of separating the portions of a mixture by heating it and condensing the components according to their different boiling points. Retreived from: http://medical-dictionary.thefreedictionary.com/fractional+distillation
Medical gas - (either a single gas or a mixture of gases) is a gas that requires no further processing in order to be administered, but is not in its final package (e.g., liquefied oxygen) and is known as a bulk gas. Retrieved from: http://ccinfoweb2.ccohs.ca/legislation/documents/stds/csa/cmgpi12e.htm
Manifold (rampe) - Equipment or apparatus designed to enable one or more medical gas containers to be filled at a time.
REFERENCES
- American Thoracic Society (2020) Clinical Practice Guideline: Home Oxygen Therapy for Adults with Chronic Lung Disease. Retrieved from: https://www.atsjournals.org/doi/pdf/10.1164/rccm.202009-3608ST
- Becker, D. E., & Casabianca, A. B. (2009). Respiratory monitoring: physiological and technical considerations. Anesthesia Progress, 56(1), 14-20. doi: 10.2344/0003-3006-56.1.14.
- Cairo, J., M. & Pilbeam, S., P., (2017) Mosby’s Respiratory Care Equipment (10th ed.). St. Louis, MO: Mosby.
- Canadian Standards Association. (2016). Z305.12-06 (R2012) - Safe Storage, Handling, and Use of Portable Oxygen Systems in Residential Buildings and Health Care Facilities. Retrieved from: https://www.csagroup.org/store/search-results/?search=all~~Safe%20Storage,%20Handling,%20and%20Use%20of%20Portable%20Oxygen%20Systems%20in%20Residential%20Buildings%20and%20Health%20Care
- Cousins JL, Wark PA, McDonald VM. Acute oxygen therapy: a review of prescribing and delivery practices. Int J Chron Obstruct Pulmon Dis. 2016;11:1067-1075. Published 2016 May 24. doi:10.2147/COPD.S103607
- Gardenshire, D. (2020). Rau’s Respiratory Care Pharmacology. (10th ed.). St. Louis, MO: Mosby Inc.
- Kacmarek, R. M., Stoller, J.K. Heuer, A. J. (2021). Egan’s Fundamentals of Respiratory Care. (12th ed.). St. Louis, MO: Mosby.
- Mariciniuk, D. D., Goodridge, D., Hemandez, P., Rocker, J., Balter, M., Bailey, P., Brown, C. (2011). Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: A Canadian Thoracic Society clinical practice guideline. Canadian Respiratory Journal, 18(2), 69–78. Retrieved from www.ncbi.nlm.nih.gov/pmc/articles/PMC3084418/
- Ministry of Health and Long-Term Care. Policy and Procedures Manual for the Assistive Devices Program (May 2016). Conflict of Interest. Retrieved from: Policies and Procedures Manual of the Assistive Devices Program (gov.on.ca)
- O'Driscoll, B. R., Howard, L. S., Earis, J., & Mak, V. (2017). British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ open respiratory research, 4(1), e000170. Retrieved from: https://doi.org/10.1136/bmjresp-2016-000170
- Sackett, D., Rosenberg, W., Gray, J., Haynes, R., & Richardson, W. (1996). Evidence-based medicine: what it is and what it isn't. British Medical Journal, 312, 71-72. Retrieved from: www.bmj.com/cgi/content/full/312/7023/71
